| SSG | Sodium Starch Glycolate |
|---|---|
| Grades | Type A (Ph. Eur.) |
| CAS NO | 9063-38-1 |
| HS Code | 29420090 |
| Molecular Formula | C2H4O3·xNa·x |
| Applications | Oral pharmaceuticals as a disintegrant in capsule and tablet formulations |
| Packing Type | 25 Kgs Corrugated boxes / Fiber Drum |
| PHARMACOPOEIAL TEST ITEMS | SPECIFICATION | REFERENCE |
|---|---|---|
| Loss on drying | Max 10.0% | Ph. Eur., NF, JP |
| pH | 5.5 - 7.5 | Ph. Eur., NF, JP |
| Residue on Ethanol | max. 3.0% | In house method |
| The raw materials, manufacturing process and product do not contain any of the solvents listed in Residual Solvents (Ph. Eur. <5.4>, USP<467>) except for ethanol limited to < 3.0% | ||
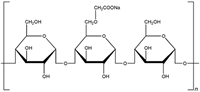
Sodium starch glycolate is the sodium salt of carboxymethyl ether. Starch glycolates are of rice, potato, wheat or corn origin. Sodium starch glycoate is a white to off-white, tasteless, odorless, relatively free flowing powder.
Sodium starch glycolate is used as a pharmaceutical grade dissolution excipient for tablets and capsules. Sodium starch glycolate absorbs water rapidly, resulting in swelling which leads to rapid disintegration of tablets and granules. It is used as a disintegrant, a suspending agent and as a gelling agent. Without a disintegrant, tablets may not dissolve appropriately and may effect the amount of active ingredient absorbed, thereby decreasing effectiveness.
|
Your message was sent successfully! I will be in touch as soon as I can.
Something went wrong, try refreshing and submitting the form again.
|